16+ Calculate The Mass Percent Of Kcl In The Solution
A solution containing 1865 g of KCl in 8135 g of water has a density of 1106 gmL. D Energy is leaving the system during reaction.

Chemistry Tips O Level Chemistry Ip Chemistry Notes By 10 Year Series Author Chemistry Specialist
What is the percent mass of potassium chloride in a solution made by adding 25 g of potassium chloride to 700 ml of water.

. What is the concentration of KOH for the final solution. C The number of moles and the mass of sodium nitrate NaNO 3 required to produce 128 g of oxygen. A 182 M b 0548 M c 0713 M d 140 M.
The percent yield is found by dividing the actual yield by the theoretical yield and then multiplying by 100. Democrats hold an overall edge across the states competitive districts. What is the molarity of the solution.
The organic ligands included are sometimes referred to as struts or linkers one example being 14-benzenedicarboxylic acid BDC. Mass solute divided. Percent yieldactual yieldtheoretical yield1000224 mol0722 mol100310 When calculating the percent yield the units of the actual and theoretical yields do not matter so long as they are the same ie both moles or both.
M 0040 moles 025 kg 016 m KCl 016 molal solution How to Calculate Normality of a Chemical Solution. A The products have a lower potential energy than the reactants. A The number of moles and the mass of chlorine Cl 2 required to react with 100 g of sodium metal Na to produce sodium chloride NaCl.
In this solution HF is-1-2-1127. Calculate the mass of solute needed to prepare 2750 mL of a physiological saline solution. Modern Analytical Chemistry Solution Manualpdf.
32 104 M b. 40 106 M. The underbanked represented 14 of US.
For example for a solution with a hydrogen ion activity of 510 6 at that level this is essentially the number of moles of hydrogen ions per litre of solution the argument of the logarithm is 1510 6 210 5. There are two types of percent concentration. Calculate the molar mass of vitamin K.
Calculate the H3O of a solution that is 020 M in HF and 010 M in NaF. The solution was found to freeze at -00943 C. Calculate the mass percent and molality of ethanol in the rum.
Calculate the percent abundances leading to the two values of the average atomic masses of boron from these two countries. More formally a metalorganic framework is a crystalline. This is the gram equivalent weight of solute per liter of solution.
Determine the limiting reactant theoretical yield of PbCl2 and percent yield for the reaction. Percent by mass and percent by volume. B The number of moles and the mass of oxygen formed by the decomposition of 1252 g of mercuryII oxide.
A lab specialist has 20 and 60 stock solutions of sodium hydroxide. Normality is similar to molarity except it expresses the number of active grams of a solute per liter of solution. C A solution formed by dissolving 075 mol of KCl in 400 kg of water.
Ficks first law can be rewritten eq 10 for the case of a nanoparticle within solution where δ is the distance from the particle surface to the bulk concentration of monomers within solution C b is the bulk concentration of monomers within the solution C i is the concentration of monomers at the solidliquid interface and C r is the. The reaction causes the temperature of the resulting solution to fall below room temperature. In a graph posted at Microsofts Activision Blizzard acquisition site the company depicts the entire gaming market as worth 165 billion in 2020 with consoles making up 33 billion 20 percent.
Thus such a solution has a pH of log 10 210 5 53. Calculate the mass of solid NaCH3COO that must be added to 10 liter of 020 M CH3COOH solution so that the pH of the resulting solution will be 500. A 0150 M sodium chloride solution is referred to as a physiological saline solution because it has the same concentration of salts as normal human blood.
The percent yield is the ratio of the actual yield to the theoretical yield expressed as a percentage. Much time and money is spent improving the percent yield for chemical. TextPercent Yield fractextActual YieldtextTheoretical Yield times 100nonumber Percent yield is very important in the manufacture of products.
Four in ten likely voters are. Percent by mass mass of solutetotal mass of solution 100 Example. What is the percentage concentration by mass of a 16molL-1 solution of nitric acid for which density is 142gmL-1.
Proposition 30 on reducing greenhouse gas emissions has lost ground in the past month with support among likely voters now falling short of a majority. Those who have a checking or savings account but also use financial alternatives like check cashing services are considered underbanked. Percent by mass mm is the mass of solute divided by the total mass of the solution multiplied by 100.
Urban High School Student. A 025 L solution with 932 g of KCl has a molarity of. Assume no volume change due to addition of solid NaCH3COO.
A student prepared a stock solution by dissolving 200 g of KOH in enough water to make 150. C The reaction is exothermic. Calculate the formula unit mass of CaCO 3 Atomic mass of C 12 u Ca 40 u O 16 u c Calculate the molecular mass of the following.
D A solution formed by dissolving 025 mol of KCl in 050 kg of water. The precipitate is filtered and dried and found to have a mass of 294 g. B This type of experiment will provide data to calculate ΔErxn.
I HNO 3 ii CH 3 COOH. E All of the solutions described have the same freezing point. Key findings include.
The actual atomic mass of boron can vary from 10807 to 10819 depending on whether the mineral source is from Turkey or the United States. To heat 500 grams of water from -160 C to 110 c-enthalpy of vaporization for water is 4056 kJmol-enthalpy for fusion of water. How do you perform a serial dilution.
Thank you so much. Comparison of Education Advancement Opportunities for Low-Income Rural vs. Calculate the value of i and estimate the percent ionization of HF in this solution.
The outcomes could determine which party controls the US House of Representatives. What is the percent by mass of a solution that contains 265 g. Transfer a portion of this solution in a conical flask as shown in figure 31.
Calculate the concentration of a solution prepared by diluting 0058 L of 028 M SiF6 solution to a volume of 0075 L. How do you calculate the molar mass of M. When 285 g KCl is added to a solution containing 257 g Pb2 a PbCl2 precipitate forms.
KCl f CaCO 3. This is 10 percent solution of the reactant. Which of the following statements is TRUE.
A A solution formed by dissolving 075 mol of KCl in 100 kg of water. Metalorganic frameworks MOFs are a class of compounds consisting of metal ions or clusters coordinated to organic ligands to form one- two- or three-dimensional structures. A 413 g B 319 g C 161 g D 877 g E 241 g.
B A solution formed by dissolving 025 mol of KCl in 100 kg of water. She then took 150 mL of the stock solution and diluted it with enough water to make 650 mL of a final solution. Modern Analytical Chemistry Solution Manualpdf.
This was exactly what I needed.

Solved What Is The Percentage By Mass Of Nacl In A Saturated Chegg Com

Solved 11 How Many Grams Of Potassium Chloride Should Be Chegg Com

R A Decarlo And P Lin Linear Circuit Analysis Solution Manuel Pdf Pdf Kilowatt Hour Series And Parallel Circuits
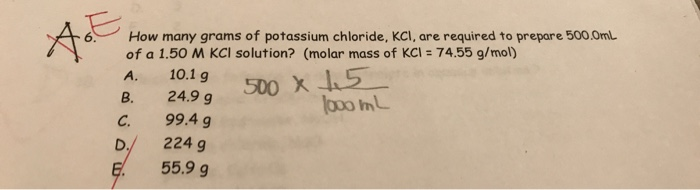
Solved How Many Grams Of Potassium Chloride Kcl Are Chegg Com
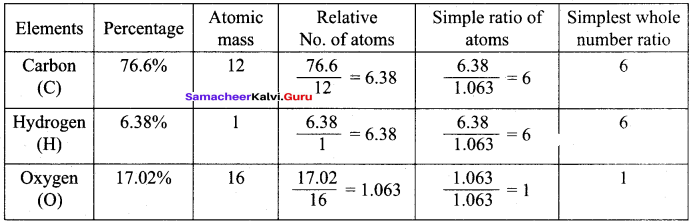
Samacheer Kalvi 11th Chemistry Solutions Chapter 1 Basic Concepts Of Chemistry And Chemical Calculations Samacheer Guru

A Solution Containing 0 5g Of Kcl Dissolved In 100g Of Water And Freezes At 0 24 Oc Calculate The Degree Of Dissociation Of The Salt Kf For Water 1 86 Oc Atomic Weights K 39 Cl 35 5
7 45 G Of Kcl Was Dissolved In 250 G Of H2o And The Solution Froze At 1 28 C Calculate Percentage Dissociation Of Salt Sarthaks Econnect Largest Online Education Community

Focus Tg4 Kssm Chemistry Terbitan Penerbitan Pelangi Sdn Bhd Flip Ebook Pages 1 48 Anyflip

Solved 1 Calculate The Mass Percent M M For 15g Of Kcl Chegg Com
If There Are 2 Grams Of Product Produced By The Chemical Reaction How Many Grams Of Reactant Were Consumed Quora
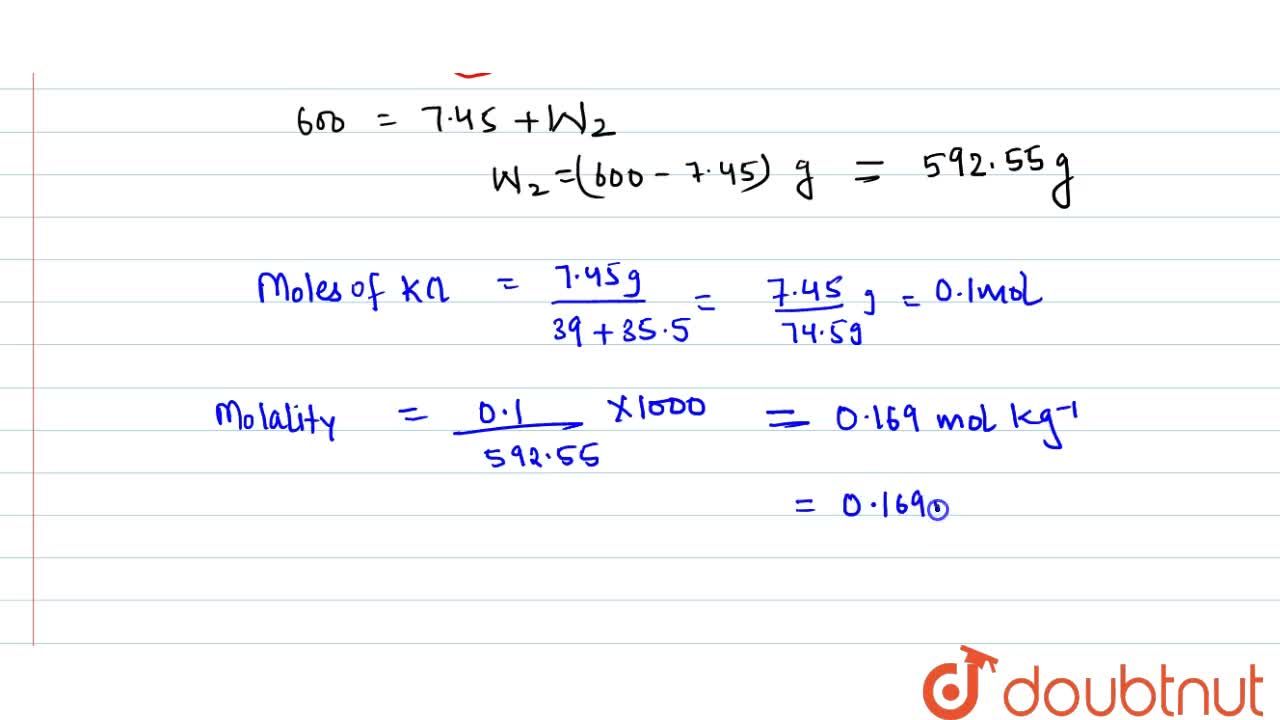
Calculate The Molarity Of Kcl Solution Prepared By Dissolving 7 45 G Of Kcl In 500 Ml Of The Solution D Sol 1 2 G Ml 1

Solution Manual Linear Circuits Analysis 2e Decarlo Pdf Electrical Network Series And Parallel Circuits
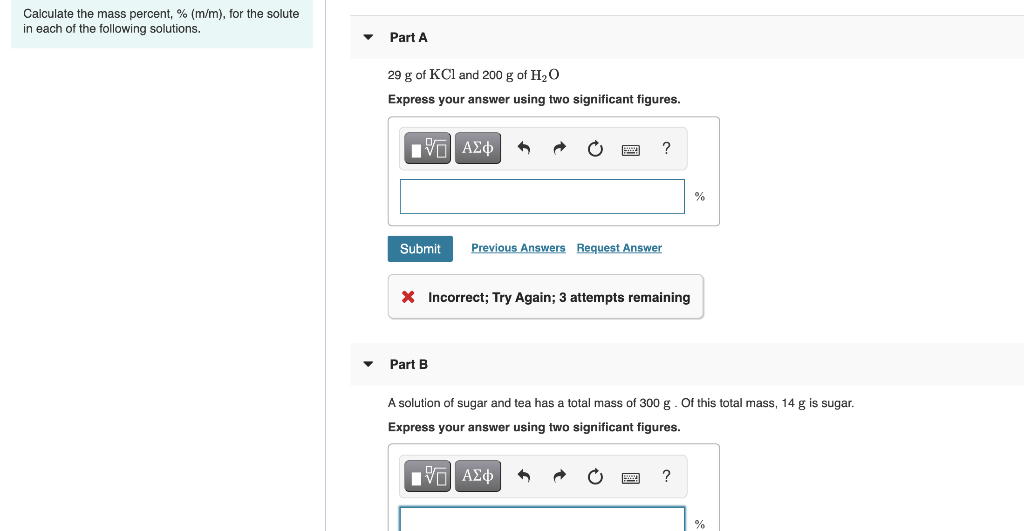
Solved Calculate The Mass Percent M M For The Solute Chegg Com

Solved Calculate The Mass Percent M M For The Solute In Each Of The Following Solutions Part A 21 G Of Kcl And 200 G Of H2o Part B 13 G

A Solution Is Prepared By Dissolving 4 G Of Naoh To Give 500 Ml Of It Calculate The Molality Of The Solution
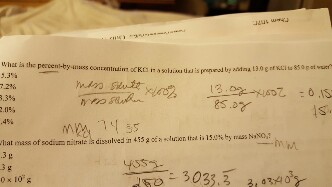
Solved What Is The Percent By Mass Concentration Of Kcl In A Chegg Com

Effect Of Muscle Electrostimulation On Afferent Activities From Tibialis Anterior Muscle After Nerve Repair By Self Anastomosis Sciencedirect